The KPIL Company is dedicated to the production of high quality pharmaceuticals products driven by our commitment to enhancing healthcare outcomes in Tanzania and across Sub Saharan Africa.
Our factory is emerging as a leading pharmaceuticals manufacturer of sterile injectables in the region setting the benchmark for excellence in quality, safety and innovation.
The factory is owned by several shareholders namely;
Kairuki Health & Education Network (KHEN), a Tanzanian social enterprise which was registered in Tanzania in 1994 and serves as a parent company for two other subsidiaries namely Kairuki Hospital and Hubert Kairuki Memorial University, other share holders include Mrs. Kokushubila Kairuki, the co-founder and current chairperson of KHEN, Kairuki Hospital (KH), which is a major shareholder as well a few other minority shareholders.
The KPIL company is limited by shares and, as such, it is open to shareholders participation, equity partners, as well as listing on the stock exchange.
Up to 1 December 2023, the project investment value stood as US 30 million dollars.

To grow and become the Pharmaceutical company of choice for the Sub-Saharan Africa region.

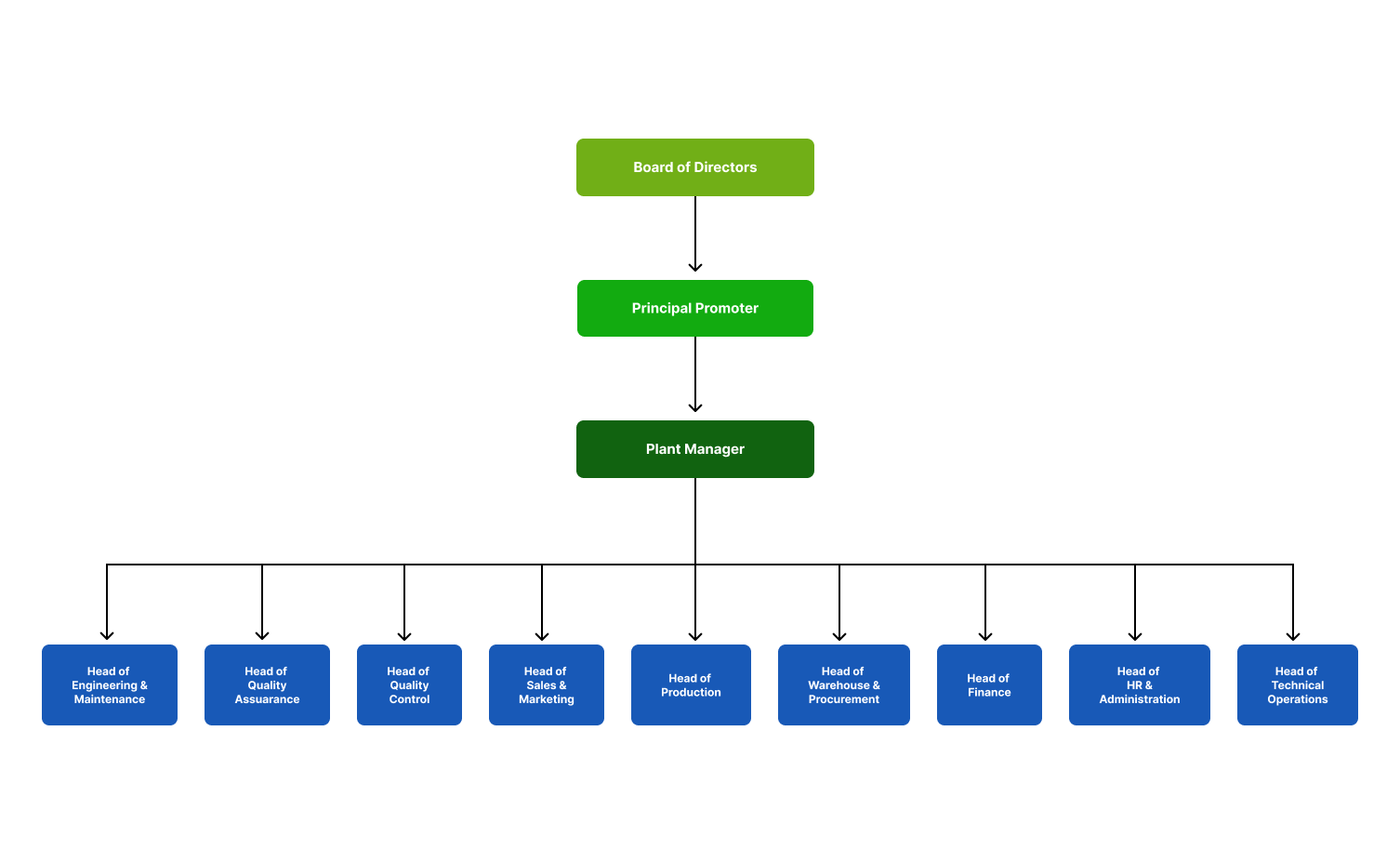
Our company is guided by an able Board of Governors and a strong management team
that steers our long-term growth strategy and ensures we are aligned to our vision and mission.
KPIL has installed the latest technology for manufacturing intravenous infusions namely the INJECTION STRETCHING BLOWING MOULDING (ISBM) system using medical grade Polypropylene plastic materials and the EUROCAP rubber stopper (single or double ports) technologies.
The ISBM technology ensures the production of High quality intravenous infusions and high packaging standards with respect to sterilisation assurance, transparency and the absence of leakage upon administration.
The Quality control laboratory at the factory and Quality Assurance systems ensures that final products are of the High standard and acceptable domestically and internationally in line with the WHO sterile manufacturing guidelines which are checked by the National Medicine Regulatory authority, TMDA.
Check through our timeline as we move towards our goal
Development of project write up, establishment and registration of company.
Acquisition of the land for the plant and site mobilisation
The Commencement of infrastructure construction following the MOU with Petra construction company & IVEN Pharma Companies
We engaged in the intricate process of acquiring a commercial loan, involving meticulous planning and diligent research.
Completion of infrastructure construction, machine installation, test runs of the installed machinery and commissioning of the plant
Commencement of the first validation batch / Trial Batch
Acquisition of GMP CERTIFICATION FROM TMDA